Lewis Dot Structure Covalent Bonds Calculator
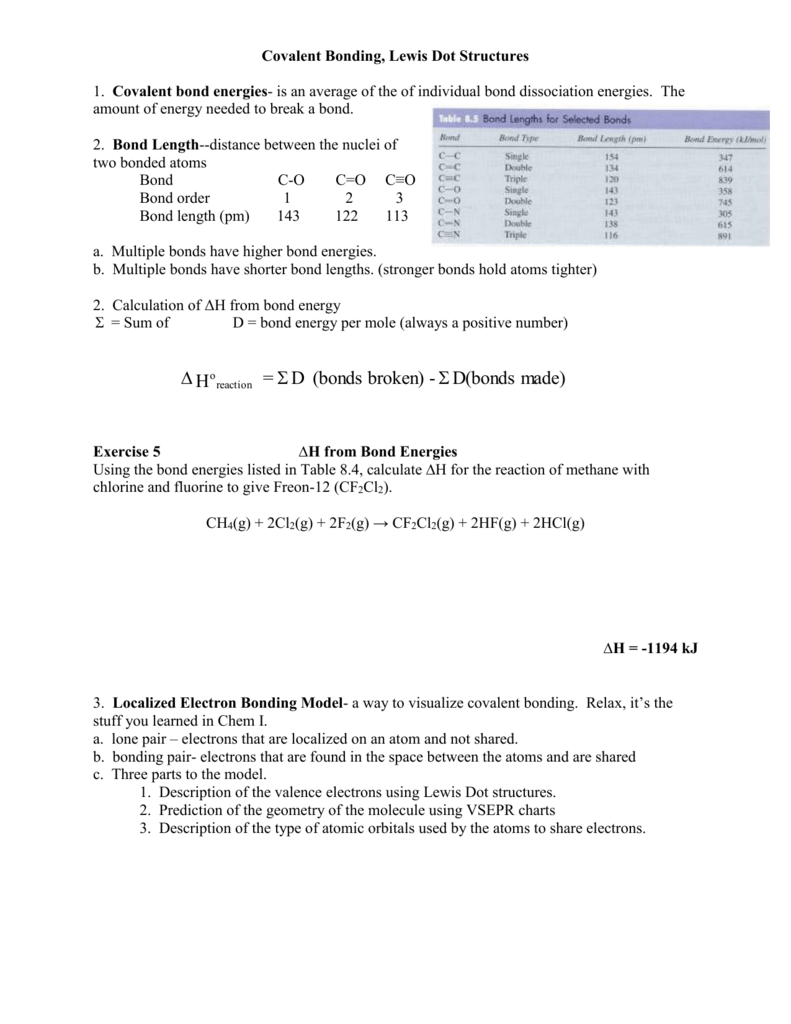
Covalent bonds are formed by nonmetals and some metalloids when they behave as nonmetals. Determine the number of valence electrons each element has, and click submit. You will then see the Lewis valence electron dot structure. Finally, determine how many more electrons they will need to complete the valence shell, and click submit again. Here is the simple view on this important topic: In Lewis structures, we show each covalent bond as two dots which represent a pair of electrons. For example: Therefore, we can say that Lewis structures are electron dot representations for molecules. The trick here is to keep track of the electrons and correctly place the atoms in the molecule. Drawing the Lewis Structure for H 3 PO 4. Viewing Notes: In the H 3 PO 4 Lewis structure Phosphorous (P) is least electron electronegative atom and goes in the center of the Lewis structure. When we have an H (or H2 or H3) in front of a polyatomic molecule (like CO 3, PO 4, NO 2, etc.) we know that it's an acid. This means that the Hydrogen.
- Lewis Dot Structure Covalent Bonds Calculator Present Value
- Lewis Dot Structure Covalent Bonds Calculator Answer
- Lewis Dot Structure Covalent Bonds
- Lewis Dot Structure For Covalent Compounds
A bond is the sharing of 2 electrons. Covalent bonds share electrons in order to form a stable octet around each atom in the molecules. Hydrogen is the exception it only requires 2 electrons (a duet) to be stable. How do we draw a covalent Lewis Dot Structure? Level 1 (basic) 1. Add up all the valance electrons of the atoms involved. ex CF4 So C has 4 and F has 7 (x4 we have 4Fs) = 32 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by F's. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 8 electrons as 4 bonds. We have 32-8= 24 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 32 electrons are now in place, count the dots around each F. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 4 bonds (2electrons) for its 8. DONE Level 2 (Double and Triple bonds) Same rules apply until #4 1. Add up all the valance electrons of the atoms involved. ex CO2 So C has 4 and O has 6 (x2 ) = 16 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by O's. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 4 electrons as 2 bonds. We have 16-4=12 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 16 electrons are now in place, count the dots around each O. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 2 bonds (2electrons) for its 4....? We need 8, so move a pair of electrons from the O to between the C and O. It will share 2 pairs of electrons instead of 1. It now has a double bond instead of a single bond.
now they all have an octet, it cleans up like this Make it symmetrical. Level 3-Lewis Dots of Polyatomic Ions Same rules apply, at the end they get brackets and a charge AP Chemistry and or College Level Rules 1. Determine whether the compound is covalent or ionic. If covalent, treat the entire molecule. If ionic, treat each ion separately. Compounds of low electronegativity metals with high electronegativity nonmetals (DEN > 1.7) are ionic as are compounds of metals with polyatomic anions. For a monoatomic ion, the electronic configuration of the ion represents the correct Lewis structure. For compounds containing complex ions, you must learn to recognize the formulas of cations and anions. 2. Determine the total number of valence electrons available to the molecule or ion by:
3. Organize the atoms so there is a central atom (usually the least electronegative) surrounded by ligand (outer) atoms. Hydrogen is never the central atom. 4. Determine a provisional electron distribution by arranging the electron pairs (E.P.) in the following manner until all available pairs have been distributed:
5. Calculate the formal charge (F) on the central atom.
6. If the central atom formal charge is zero or is equal to the charge on the species, the provisional electron distribution from (4) is correct. Calculate the formal charge of the ligand atoms to complete the Lewis structure. 7. If the structure is not correct, calculate the formal charge on each of the ligand atoms. Then to obtain the correct structure, form a multiple bond by sharing an electron pair from the ligand atom that has the most negative formal charge.
8. Recalculate the formal charge of each atom to complete the Lewis structure. on to Formal Charge Chemical Demonstration Videos |
Formal charge:
Let us draw the Lewis structure for carbon dioxide.
1. Skeletal structure
2. Total number of valence electrons in CO2
=[1 x 4(carbon)] +[2 x 6(oxygen)] = 4+ 12 = 16
3. Draw single bonds between atoms. Two bonds can be drawn as shown in the figure for CO2 which accounts for four electrons (2 bond pairs).
4. Distribute the remaining twelve electrons (16 - 4= 12) as six lone pairs starting from most electronegative atom, the oxygen. Six lone pairs are distributed to the two terminal oxygens (three each) to satisfy their octet.
5. Verify weather all the atoms have octet configuration. In the above distribution, the central carbon has two pair short for octet. Therefore, to satisfy the octet rule two lone pairs from one oxygen or one pair from each oxygen can be moved to form multiple bonds, leading the formation of two possible structures for carbon dioxide as shown below
Similarly, the Lewis structure for many molecules drawn using the above steps gives more than one acceptable structure. Let us consider the above mentioned two structures of carbon dioxide.
Which one the above forms represents the best distribution of electrons in the molecule. To find an answer, we need to know the formal charge of each atom in the Lewis structures. Formal charge of an atom in a molecule, is the electrical charge difference between the valence electron in an isolated atom and the number of electrons assigned to that atom in the Lewis structure.
Where,
Nv- Number of valence electron of atom in its isolated state.
Lewis Dot Structure Covalent Bonds Calculator Present Value
Nl - Number of electrons present as lone pairs around the atom in the Lewis structure
Nb - Number of electrons present in bonds around the atom (bond pairs) in the Lewis structure]
Now let us calculate the formal charge on all atoms in both structures,
For Structure 1,
For structure 2
Formal charge on carbon
After calculating the formal charges, the best representation of Lewis structure can be selected by using following guidelines.
Lewis Dot Structure Covalent Bonds Calculator Answer
1. A structure in which all formal charges are zero preferred over the one with charges.
2. A structure with small formal charges is preferred over the one with higher formal charges.
Lewis Dot Structure Covalent Bonds
3. A structure in which negative formal charges are placed on the most electronegative atom is preferred.
Lewis Dot Structure For Covalent Compounds
In case of CO2 structures, the structure one is preferred over the structure 2 as it has zero formal charges for all atoms.